
Privacy statement: Your privacy is very important to Us. Our company promises not to disclose your personal information to any external company with out your explicit permission.
This article aims to provide a comprehensive exploration of galvanization. We will equip readers with a thorough understanding of this critical corrosion protection technique.
The efficacy of galvanization lies in a combination of barrier protection and cathodic protection, leveraging the electrochemical properties of zinc in relation to steel.
1. Barrier Protection: The most immediate and apparent function of the zinc coating is to act as a physical barrier, isolating the underlying steel or iron from the corrosive elements in the environment, such as moisture, oxygen, and pollutants. This barrier prevents direct contact between the steel surface and these corrosive agents, thus inhibiting the electrochemical reactions that lead to rust formation.
2. Cathodic Protection (Sacrificial Anode): The true genius of galvanization, however, resides in its ability to provide cathodic protection. This principle is rooted in the electrochemical series, which ranks metals based on their electrode potential. Zinc is more electrochemically active (more negative standard electrode potential) than iron. This means that when zinc and steel are in electrical contact in the presence of an electrolyte (like water or moisture), zinc will preferentially corrode.
In essence, zinc acts as a sacrificial anode. If the galvanized coating is scratched or damaged, exposing the underlying steel, the zinc surrounding the exposed area will corrode preferentially to the steel. This corrosion of zinc releases electrons, which flow to the steel, making the steel surface cathodic. By becoming cathodic, the steel is protected from oxidation (rusting), as the electrochemical reaction that causes corrosion is suppressed on the steel surface and concentrated on the zinc.
The electrochemical reactions can be simplified as follows:
a. Anodic Reaction (Zinc Corrosion): Zinc atoms lose electrons and become zinc ions, dissolving into the electrolyte.
b. Cathodic Reaction (Oxygen Reduction in Neutral or Alkaline Solutions): Oxygen and water react with electrons to form hydroxide ions.
c. Cathodic Reaction (Hydrogen Evolution in Acidic Solutions): Hydrogen ions react with electrons to form hydrogen gas.
The electrons released from the zinc oxidation are consumed in the cathodic reactions, preventing the iron from undergoing oxidation (rusting):
d. Iron Oxidation (Rusting - prevented by cathodic protection): Iron atoms would normally lose electrons and react with oxygen and water to form hydrated iron(III) oxide (rust), but this is suppressed due to the cathodic protection provided by zinc.
The corrosion products of zinc, primarily zinc oxides and carbonates, are relatively insoluble and adhere well to the surface, further contributing to the barrier protection and slowing down the corrosion process. This self-healing mechanism, where zinc continues to protect even when the coating is locally damaged, is a key advantage of galvanization.

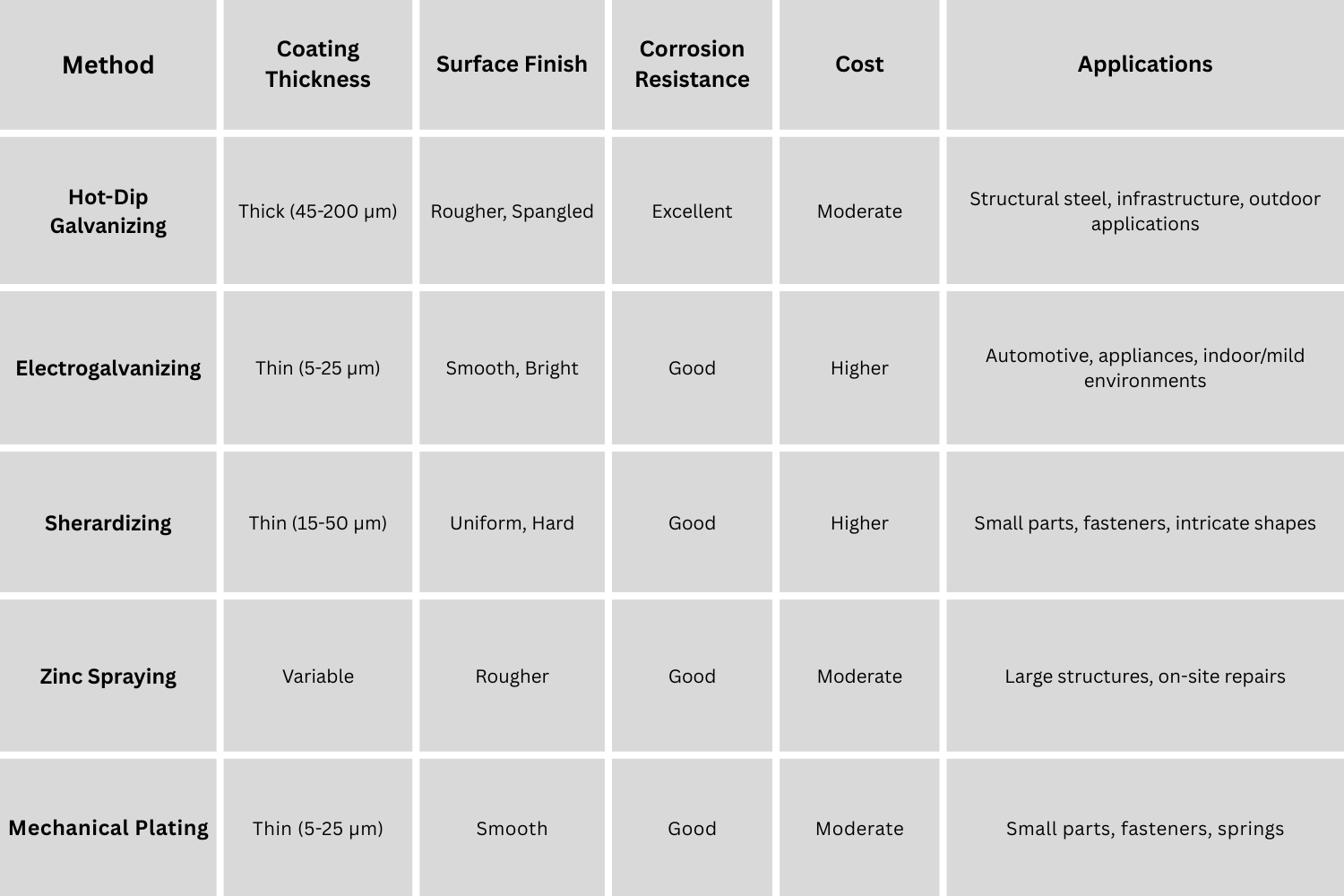
While the fundamental principle of applying a zinc coating remains consistent, various galvanization methods have been developed to suit different applications, material types, and desired coating characteristics. The most common methods employed in manufacturing are:
1. Hot-Dip Galvanizing: This is the most widely used galvanization process, particularly for structural steel and large components. It involves immersing clean steel in a bath of molten zinc at a temperature of around 450°C (842°F). The process typically involves the following steps:
a. Surface Preparation: This is crucial for achieving a high-quality coating. It usually includes:
i. Degreasing: Removing oil, grease, and dirt from the steel surface using alkaline solutions or solvents.
ii. Pickling: Removing mill scale and rust using acid solutions, typically hydrochloric or sulfuric acid.
iii. Rinsing: Thoroughly rinsing the steel to remove residual acid.
iv. Fluxing: Applying a flux, often zinc ammonium chloride, to the steel surface. The flux serves multiple purposes: it prevents oxidation of the steel surface before immersion in the zinc bath, removes any remaining oxides, and promotes wetting of the steel by the molten zinc, ensuring a uniform and adherent coating.
b. Galvanizing (Immersion): The prepared steel is then immersed in the molten zinc bath. A metallurgical reaction occurs at the steel-zinc interface, forming a series of zinc-iron alloy layers. These layers are harder than pure zinc and are integral to the coating's durability and abrasion resistance. The coating thickness is primarily controlled by the immersion time and the steel's reactivity.
c. Withdrawal and Cooling: The galvanized steel is slowly withdrawn from the zinc bath, allowing excess zinc to drain off. The coated component is then cooled, usually in air or a water quench. Air cooling allows for the formation of a brighter, spangled finish, while water quenching can be used to reduce reactivity and prevent excessive alloy layer growth in certain steel grades.
Hot-dip galvanizing produces a relatively thick, robust coating that provides excellent corrosion protection, especially in harsh environments. It is suitable for a wide range of steel products, from small fasteners to large structural members.
2. Electrogalvanizing: Also known as electrolytic galvanizing, this method uses an electrolytic process to deposit a thin layer of zinc onto the steel. The steel component is immersed in an electrolyte solution containing zinc salts and acts as the cathode in an electrochemical cell. Zinc anodes are also immersed in the solution. When an electric current is passed through the cell, zinc ions from the electrolyte are reduced at the cathode (steel surface) and deposited as a zinc coating.
a. Process Characteristics: Electrogalvanizing typically produces a much thinner and smoother zinc coating compared to hot-dip galvanizing. The coating thickness is precisely controlled by adjusting the current density and plating time. Electrogalvanized coatings are generally brighter and more aesthetically appealing than hot-dip coatings.
b. Applications: Due to the thinner coating, electrogalvanizing is often used for applications where appearance is important and corrosion conditions are less severe, such as:
i. Automotive body panels and components
ii. Appliances
iii. Furniture
iv. Electrical conduits and hardware
Electrogalvanizing is less suitable for heavy-duty applications requiring long-term corrosion protection in aggressive environments compared to hot-dip galvanizing.
3. Other Galvanization Methods (Less Common in Manufacturing):
a. Sherardizing (Vapor Galvanizing): Steel parts are tumbled in a rotating drum with zinc dust and heated to around 370-500°C (700-930°F) in an oxygen-free atmosphere. Zinc vapor diffuses into the steel surface, forming a zinc-iron alloy coating. Sherardizing produces a uniform, thin, and hard coating, particularly suitable for small, intricate parts like fasteners and hardware.
b. Zinc Spraying (Metallizing): Molten zinc is sprayed onto the steel surface using a spray gun. This method is often used for on-site galvanizing of large structures or for repairing damaged galvanized coatings. The coating is purely mechanical and does not form alloy layers like hot-dip galvanizing.
c. Mechanical Plating (Pechardizing): Steel parts are tumbled in a rotating barrel with zinc powder, glass beads, and chemical promoters. The impact of the glass beads mechanically welds the zinc powder onto the steel surface. This method produces a uniform, thin coating, primarily used for small parts like fasteners and springs.
The choice of galvanization method depends on factors such as the size and shape of the component, required coating thickness, desired surface finish, application environment, and cost considerations. For manufacturing applications demanding robust and long-lasting corrosion protection, hot-dip galvanizing remains the dominant and often preferred method.
Galvanization is primarily applied to steel and iron to protect them from corrosion. The process is most effective on low-carbon and mild steels, which are commonly used in manufacturing and construction.
● Steel Grades: Various steel grades can be galvanized, including:
○ Mild Steel: The most common type of steel galvanized, offering excellent results with hot-dip galvanizing.
○ Structural Steel: Widely galvanized for buildings, bridges, and infrastructure projects.
○ High-Strength Low-Alloy (HSLA) Steels: Can be galvanized, but require careful process control, particularly in hot-dip galvanizing, to avoid hydrogen embrittlement.
○ Certain Alloy Steels: Some alloy steels can be galvanized, but the presence of certain alloying elements (like silicon or phosphorus in higher concentrations) can affect the reactivity with zinc and the coating quality. Steels with high silicon content (Killed steels with silicon levels above 0.04% or silicon-killed steels above 0.12%) can exhibit accelerated coating growth and potentially brittle coatings in hot-dip galvanizing, known as the Sandelin effect.
● Cast Iron: Cast iron can also be galvanized, although the process may be more challenging due to the higher silicon content and surface roughness of cast iron. Hot-dip galvanizing is generally feasible, but careful surface preparation and process control are necessary.
● Limitations with Other Metals: Galvanization is not typically applied to other metals like aluminum, stainless steel, or copper. These metals have their own inherent corrosion resistance or are protected by other methods. Applying zinc coatings to these metals is generally not effective or necessary.
Galvanization offers a multitude of benefits, making it a highly attractive corrosion protection solution for manufacturing and various industries:
1. Long-Term Corrosion Protection: Galvanized coatings provide decades of maintenance-free corrosion protection in many environments. The combination of barrier and cathodic protection ensures long-lasting durability, significantly extending the service life of steel structures and components. The lifespan of a galvanized coating is directly proportional to its thickness and the severity of the environment. In typical atmospheric conditions, hot-dip galvanized steel can last for 20 to 50 years or even longer without requiring maintenance.
2. Low Initial Cost and Life-Cycle Cost Effectiveness: While the initial cost of galvanizing might be slightly higher than some other coatings like paint in certain cases, galvanization is highly cost-effective over the long term. The extended lifespan and minimal maintenance requirements of galvanized steel result in significantly lower life-cycle costs compared to systems requiring frequent repainting or replacement due to corrosion.
3. Low Maintenance: Galvanized steel is virtually maintenance-free. Once galvanized, the coating typically requires no further treatment or maintenance for decades. This eliminates the need for costly and time-consuming inspections, repairs, and recoating, especially in hard-to-reach or critical locations.
4. Sacrificial Protection - Self-Healing: As discussed earlier, the sacrificial nature of zinc provides inherent self-healing properties. Even if the galvanized coating is scratched or damaged, the surrounding zinc continues to protect the exposed steel from corrosion. This is a significant advantage over barrier coatings like paint, where damage can lead to localized corrosion that spreads rapidly.
5. Recyclability: Both steel and zinc are highly recyclable materials. Galvanized steel can be recycled without removing the zinc coating, and zinc itself can be recovered and reused. This contributes to the sustainability and environmental friendliness of galvanization.
Galvanization is a ubiquitous corrosion protection method across a vast array of industries, particularly within the manufacturing sector. Its versatility, durability, and cost-effectiveness make it indispensable for numerous applications:
1. Construction and Infrastructure: This is one of the largest application areas for galvanization.
a. Structural Steel: Beams, columns, trusses, and other structural members for buildings, bridges, stadiums, and industrial facilities are extensively hot-dip galvanized for long-term durability and safety.
b. Bridges and Highways: Bridge components, guardrails, signposts, lighting poles, and other highway infrastructure elements are often galvanized to withstand harsh weather conditions and de-icing salts.
c. Transmission Towers and Utility Poles: Galvanized steel towers and poles are used for power transmission, telecommunications, and utility distribution networks, ensuring reliable performance in outdoor environments.
d. Pipelines and Water Infrastructure: Galvanized steel pipes are used for water distribution, sewage systems, and irrigation, providing corrosion resistance in buried and submerged conditions.
2. Automotive Industry: Electrogalvanizing is widely used in the automotive industry for:
a. Car Body Panels: Provides corrosion protection and a smooth surface for painting.
b. Chassis Components: Frames, suspension parts, and other chassis components are often galvanized for durability and safety.
c. Fasteners and Hardware: Bolts, nuts, screws, and other fasteners are frequently galvanized to prevent rust and ensure reliable assembly.
3. Agriculture: Galvanization plays a crucial role in agricultural applications:
a. Fencing: Galvanized steel wire and mesh are used for livestock fencing, perimeter fencing, and agricultural enclosures, providing long-lasting protection against weather and animal wear.
b. Farm Equipment: Components of tractors, trailers, planters, harvesters, and other farm machinery are often galvanized to withstand harsh outdoor conditions and exposure to fertilizers and chemicals.
c. Greenhouse Structures: Galvanized steel frames are used for greenhouse construction, providing structural support and corrosion resistance in humid environments.
4. Manufacturing Equipment and Machinery: Galvanization protects critical components in various manufacturing settings:
a. Conveyor Systems: Frames, rollers, and supports for conveyor systems in warehouses, factories, and distribution centers are often galvanized for durability and reduced maintenance.
b. Storage Racks and Shelving: Galvanized steel racks and shelving are used in warehouses, workshops, and retail environments, providing robust and corrosion-resistant storage solutions.
c. Machine Guards and Safety Barriers: Galvanized steel guards and barriers are used to protect machinery and personnel in industrial settings, ensuring safety and longevity.
d. Process Equipment: Certain components of chemical processing equipment, food processing machinery, and other Industrial Equipment are galvanized to resist corrosion from process fluids and cleaning agents.

The versatility and effectiveness of galvanization ensure its continued importance as a primary corrosion protection method across a wide spectrum of manufacturing and industrial applications, contributing to the longevity, reliability, and sustainability of countless products and structures.
Against the backdrop of manufacturing transformation, the precision flexible intelligent manufacturing platform has become a core support in high-end fields. This article details its definition, pain point solutions, and industry applications, helping enterprises understand the platform's value.
In the manufacturing of life science components and medical equipment parts, welding is a key factor in quality. Traditional manual welding struggles to meet high-precision requirements, and robotic arm welding, with its automation advantages, has become a core solution in the industry.
Precision planing and slotting use digital control to improve accuracy and consistency, and are widely used in medical and life science manufacturing.
Life science and medical device manufacturing often suffers from slow changeovers and low utilization in mixed-product lines. Flexible intelligent platforms with smart scheduling enable multi-product production on one line, boosting efficiency and utilization.
Email to this supplier

Privacy statement: Your privacy is very important to Us. Our company promises not to disclose your personal information to any external company with out your explicit permission.